Gibson Assembly® Master Mix
Product information| Code | Name | Size | Quantity | Price | |
|---|---|---|---|---|---|
E2611S |
Gibson Assembly Master Mix |
10 rxns | - | Unavailable in your region | |
E2611L |
Gibson Assembly Master Mix |
50 rxns | - | Unavailable in your region |
Gibson Assembly® Master Mix
Gibson Assembly Master Mix has been reformulated with components containing Recombinant Albumin (rAlbumin) beginning with Lot #10229799. Learn more.
Product Introduction
Gibson Assembly allows for successful assembly of multiple DNA fragments, regardless of fragment length or end compatibility.
- Assembly and transformation in just under two hours
- Flexible sequence design (scar-less cloning)
- No PCR clean-up step required
- High transformation efficiencies for inserts up to 20 kb
- Easily adapted for multiple DNA manipulations, including site-directed mutagenesis
| Catalog # | Size | Concentration |
|---|---|---|
| E2611S | 10.0 reactions | |
| E2611L | 50.0 reactions |
Featured Videos
View Video Library-

Introduction to Gibson Assembly®
-
Gibson Assembly Workflow
-

NEBUILDER® Assembly Tool 2.0 What’s New?
-
NEBUILDER® Assembly Tool 2.0 Fragments Amplified by PCR
-

NEBUILDER® Assembly Tool 2.0 Restriction Enzyme Digest
-
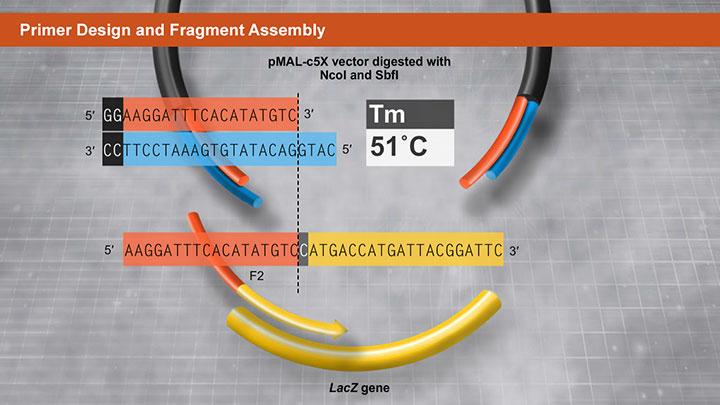
Primer Design and Fragment Assembly Using NEBuilder HiFi DNA Assembly® or Gibson Assembly®
- Product Information
- Protocols, Manuals & Usage
- Tools & Resources
- FAQs & Troubleshooting
- Citations & Technical Literature
- Quality, Safety & Legal
- Other Products You May Be Interested In
Product Information
Description
Gibson Assembly was developed by Dr. Daniel Gibson and his colleagues at the J. Craig Venter Institute and licensed to NEB by Synthetic Genomics, Inc. It allows for successful assembly of multiple DNA fragments, regardless of fragment length or end compatibility. It has been rapidly adopted by the synthetic biology community due to its ease-of-use, flexibility and suitability for large DNA constructs.
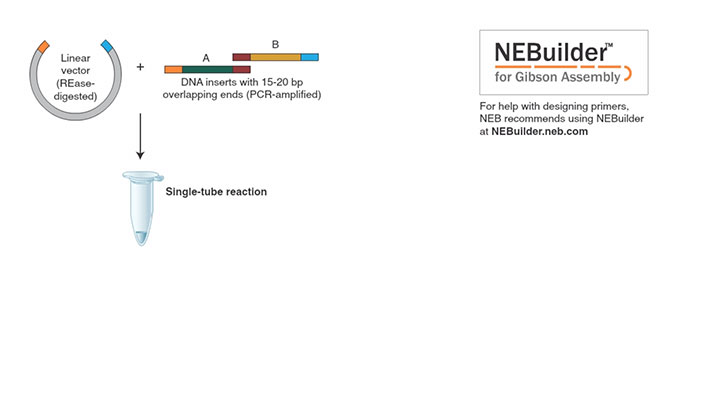
Gibson Assembly efficiently joins multiple overlapping DNA fragments in a single-tube isothermal reaction (1,2). The Gibson Assembly Master Mix includes three different enzymatic activities that perform in a single buffer:
- The exonuclease creates single-stranded 3´ overhangs that facilitate the annealing of fragments that share complementarity at one end (overlap region).
- The polymerase fills in gaps within each annealed fragment.
- The DNA ligase seals nicks in the assembled DNA.
To help select the best DNA assembly method for your needs, please use our Synthetic Biology/DNA Assembly Selection Chart.
For help designing primers, please view our primer design video.
Overview of the Gibson Assembly Method

Specification:
10 μl of 2X Gibson Assembly Master Mix was incubated with 6 fragments (5 fragments of 400 bp and one of 2,780 bp, with 40 bp overlap, 0.05 pmol each) in a final volume of 20 μl at 50°C for 60 minutes. NEB 5-alpha Competent E. coli (NEB #C2987) were transformed with 2 μl of the master mix/fragment mixture using the transformation protocol. Greater than 100 white colonies were observed when 1/10 of the outgrowth was spread on an ampicillin plate with IPTG/Xgal and incubated overnight.Overview of Gibson Assembly Master Mix Protocol:
- Design primers to amplify fragments (and/or vector) with appropriate overlaps
- PCR amplify fragments using a high-fidelity DNA polymerase.
- Prepare linearized vector by PCR amplification using a high-fidelity DNA polymerase or by restriction digestion.
- Confirm and determine concentration of fragments using agarose gel electrophoresis, a Nanodrop™ instrument or other method
- Add DNAs to Gibson Assembly Master Mix and incubate at 50°C for 15 minutes to 1 hour, depending on number of fragments being assembled.
- Transform into E. coli or use directly in other applications
- This product is related to the following categories:
- DNA Assembly, Cloning and Mutagenesis Kits Products
- This product can be used in the following applications:
- Gibson Assembly®
Kit Components
Kit Components
The following reagents are supplied with this product:
| NEB # | Component Name | Component # | Stored at (°C) | Amount | Concentration | |
|---|---|---|---|---|---|---|
Properties & Usage
Materials Required but not Supplied
DNA Polymerases (for generating PCR products):We recommend Q5® High-Fidelity DNA Polymerase (NEB #M0491) or related products, such as Q5 Hot Start Flex DNA Polymerase (NEB #M0493), Q5 Hot Start Flex 2X Master Mix (NEB #M0494).
LB (Luria-Bertani) plates with appropriate antibiotic.
SOC Outgrowth Medium (NEB #B9020).
Competent Cells:
We recommend NEB 5-alpha Competent E. coli (High Efficiency, NEB #C2987). For assembled products greater than 10 kb, NEB recommends using NEB 10-beta Competent E. coli (High Efficiency, NEB #C3019) or NEB 10-beta Electrocompetent E. coli (NEB #C3020).
Storage Notes
- Store at -20°C. Thaw, vortex thoroughly before use and keep on ice.
Features
- Increased number of successful assembly products, particularly for longer or greater number of fragments
- Flexible sequence design (scarless cloning)
- No clean-up step required
- Complex assembly achieved in 1 hour
- DNA can be used immediately for transformation, or as template for PCR or RCA
- Easily adapted for multiple DNA manipulations, including site directed mutagenesis, insertions and deletions
Related Products
Companion Products
- NEB® 10-beta Competent E. coli (High Efficiency)
- NEB® 10-beta Electrocompetent E. coli
- NEB® 5-alpha Competent E. coli (High Efficiency)
- SOC Outgrowth Medium
- Gibson Assembly® Cloning Kit
- Q5® Hot Start High-Fidelity 2X Master Mix
- Q5® Hot Start High-Fidelity DNA Polymerase
- Q5® High-Fidelity 2X Master Mix
- Q5® High-Fidelity DNA Polymerase
- Monarch® Plasmid Miniprep Kit
- Monarch® DNA Gel Extraction Kit
- Monarch® PCR & DNA Cleanup Kit (5 μg)
Product Notes
- General notes:
We highly recommend using our web tool, NEBuilder™ to design PCR primers with overlapping sequences between the adjacent DNA fragments and for their assembly iinto a cloning vector. - Usage notes:
To ensure the successful assembly and subsequent transformation of assembled DNAs, NEB recommends the following:- Cells: Transformation efficiency of competent cells can vary by several logs. Perceived assembly efficiency directly correlates to the competence of the cells used for transformation.
- Electroporation: Electroporation can increase transformation efficiency by several logs. When using the Gibson Assembly Master Mix product for electroporation, it is necessary to dilute the reaction 3-fold and use 1 μl for transformation.
- DNA: PCR product purification is not necessary if the total volume of all PCR products in the Gibson Assembly reaction is 20% or less of the Gibson Assembly reaction volume. Higher volumes of PCR products may reduce the efficiency of Gibson Assembly and transformation due to the elevated carryover amounts of PCR reaction buffer and unused primers present in the PCR product. Column purification of PCR products may increase the efficiency of both Gibson Assembly and transformation by 2–10 fold and is highly recommended when performing assemblies of three or more PCR fragments or assembling longer than 5 kb fragments. Purified DNA for assembly can be dissolved in ddH2O (Milli-Q® water or equivalent is preferable), TE or other dilution buffers.
- Insert: When directly assembling fragments into a cloning vector, the concentration of assembly fragments should be 2–3 times higher than the concentration of vector. For assembly of 3 or more fragments, we recommend using equilmolar ratio of fragments.
- Biology: Some DNA structures, including inverted and tandem repeats, are selected against by E. coli. Some recombinant proteins are not well tolerated by E. coli and can result in poor transformation or small colonies.
References
- Gibson, D.G. et.al (2009). NatureMethods. 343-345.
- Gibson, D.G. et al. (2010). NatureMethods. 901-903.
- Barnes, W.M. (1994). Proc. Natl. Acad. Sci.. 91, 2216-2220.
Protocols, Manuals & Usage
Protocols
Manuals
Application Notes
Tools & Resources
Selection Charts
Web Tools
FAQs & Troubleshooting
FAQs
- I am not sure whether to choose NEBuilder HiFi DNA Assembly or NEB Gibson Assembly? How are the products different?
- What are the advantages of this method compared to traditional cloning methods?
- How large a DNA fragment can I assemble?
- How many fragments of DNA can be assembled in one reaction?
- What are the shortest overlaps that can be used with this assembly method?
- What are the longest overlaps that can be used with this method?
- Can ≤ 200 bp dsDNA fragments be assembled by this method?
- Can ssDNA oligonucleotides be combined and assembled with dsDNA fragments?
- Can longer or shorter incubation times be used?
- Will the reaction work at other temperatures?
- Is it necessary to inactivate restriction enzymes after vector digestion?
- I would like to produce overlapping dsDNA fragments by PCR. Do I need to use PCR primers that have been purified by PAGE or HPLC?
- I would like to assemble ssDNA oligonucleotides into dsDNA fragments. Do I need to use oligonucleotides that have been purified by PAGE or HPLC?
- Can I use a 15-nt overlap that is entirely composed of His-tag repeats (i.e. CACCACCACCACCAC)?
- Can you PCR-amplify the assembled product?
- What should I do if my assembly reaction yields no colonies, a small number of colonies, or clones with the incorrect insert size following transformation into E. coli?
- How can I reduce the number of vector-only background colonies?
- What type of competent cells are suitable for transformation of DNA constructs created using Gibson Assembly?
- Can I use electroporation instead of chemical transformation?
- Are there any differences between the Gibson Assembly Master Mix (NEB #E2611) and Gibson Assembly Master Mix included in the Gibson Assembly Cloning Kit (NEB #E5510)?
- Are there any differences between the requirements for 2-3 fragmentassemblies versus 4–6?
- The Gibson Assembly Master Mix control reaction is not giving me any colonies. Why?
- When using a polymerase that doesn't contain a 3'-5' exonuclease activity (such as Taq DNA Polymerase) to amplify fragments to be used in a Gibson Assembly reaction, should I be concerned about the potential 3' mismatch generated by the addition of a non-templated nucleotide?
- Is storing Gibson Assembly Master Mix at -80°C harmful?
- I would like to use NEBuilder but am concerned about user data privacy. How does NEB handle the information that I enter into NEBuilder?
- Can I Use other primer design tools such as SnapGene for Gibson Assembly, to design primers for NEBuilder HiFi DNA Assembly?
- What antibiotic resistance is encoded in the NEBuilder Positive Control (assembly control)?
Citations & Technical Literature
Citations
Additional Citations
- Law SH, Sargent TD (2014) The Serine-Threonine Protein Kinase PAK4 Is Dispensable in Zebrafish: Identification of a Morpholino-Generated Pseudophenotype PLoS One; 9(6), e100268. PubMedID: 24945275, DOI: 10.1371/journal.pone.0100268
- Lipscomb GL, Schut GJ, Thorgersen MP, Nixon WJ, Kelly RM, Adams MW (2014) Engineering hydrogen gas production from formate in a hyperthermophile by heterologous production of an 18-subunit membrane-bound complex J Biol Chem; 289(5), 2873-9. PubMedID: 24318960, DOI: 10.1074/jbc.M113.530725
- Guilinger JP, Thompson DB, Liu DR (2014) Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification Nat Biotechnol; 32(6), 577-82. PubMedID: 24770324, DOI: 10.1038/nbt.2909
- Gai CS, Lu J, Brigham CJ, Bernardi AC, Sinskey AJ (2014) Insights into bacterial CO2 metabolism revealed by the characterization of four carbonic anhydrases in Ralstonia eutropha H16 AMB Express; 4(1), 2. PubMedID: 24410804, DOI: 10.1186/2191-0855-4-2
- Vandergaast R, Hoover LI, Zheng K, Fredericksen BL (2014) Generation of West Nile virus infectious clones containing amino acid insertions between capsid and capsid anchor Viruses; 6(4), 1637-53. PubMedID: 24721788, DOI: 10.3390/v6041637
- Phelan VV, Moree WJ, Aguilar J, Cornett DS, Koumoutsi A, Noble SM, Pogliano K, Guerrero CA, Dorrestein PC (2014) Impact of a transposon insertion in phzF2 on the specialized metabolite production and interkingdom J Bacteriol; 196(9), 1683-93. PubMedID: 24532776, DOI: 10.1128/JB.01258-13
- Chinnici JL, Fu C, Caccamise LM, Arnold JW, Free SJ (2014) Neurospora crassa Female Development Requires the PACC and Other Signal Transduction Pathways, Transcription Factors, Chromatin Remodeling, Cell-To-Cell Fusion, and Autophagy PLoS One; 9(10), e110603. PubMedID: 25333968, DOI: 10.1371/journal.pone.0110603
Quality, Safety & Legal
Quality Assurance Statement
Quality Control tests are performed on each new lot of NEB product to meet the specifications designated for it. Specifications and individual lot data from the tests that are performed for this particular product can be found and downloaded on the Product Specification Sheet, Certificate of Analysis, data card or product manual. Further information regarding NEB product quality can be found here.Specification Change Notifications
Gibson Assembly Master Mix has been reformulated with components containing Recombinant Albumin (rAlbumin) beginning with Lot #10229799. All subsequent (higher number) lots will contain rAlbumin. For additional information, please visit at NEB Restriction Enzyme formulations with Recombinant Albumin (rAlbumin).
Specifications
The Specification sheet is a document that includes the storage temperature, shelf life and the specifications designated for the product. The following file naming structure is used to name these document files: [Product Number]_[Size]_[Version]Certificate Of Analysis
The Certificate of Analysis (COA) is a signed document that includes the storage temperature, expiration date and quality controls for an individual lot. The following file naming structure is used to name these document files: [Product Number]_[Size]_[Version]_[Lot Number]- E2611S_L_v1_0271802
- E2611L_v1_10008765
- E2611S_v1_10008766
- E2611S_v1_10016555
- E2611L_v1_10020271
- E2611S_v1_10023746
- E2611L_v1_10025028
- E2611S_v1_10027193
- E2611L_v1_10030518
- E2611S_v1_10032255
- E2611L_v1_10037180
- E2611S_v1_10037181
- E2611L_v1_10041160
- E2611S_v1_10041604
- E2611L_v1_10045951
- E2611S_v1_10047464
- E2611S_v1_10049696
- E2611L_v1_10047465
- E2611S_v1_10053866
- E2611L_v1_10056362
- E2611S_v1_10058279
- E2611S_v1_10060511
- E2611L_v1_10060510
- E2611S_v1_10062690
- E2611L_v1_10068229
- E2611S_v1_10069216
- E2611L_v1_10072379
- E2611S_v1_10072378
- E2611S_v1_10075861
- E2611L_v1_10080103
- E2611S_v1_10080104
- E2611L_v1_10083380
- E2611S_v1_10084893
- E2611S_v1_10091343
- E2611S_v1_10095426
- E2611L_v1_10094125
- E2611S_v1_10098672
- E2611S_v1_10105387
- E2611L_v1_10105386
- E2611S_v1_10109414
- E2611L_v1_10112963
- E2611S_v1_10117464
- E2611L_v1_10117466
- E2611S_v1_10120085
- E2611L_v1_10122045
- E2611S_v1_10128996
- E2611L_v1_10130260
- E2611S_v1_10133466
- E2611L_v1_10138494
- E2611S_v1_10144142
- E2611L_v1_10146835
- E2611S_v1_10148887
- E2611L_v1_10148890
- E2611S_v1_10155114
- E2611L_v1_10160323
- E2611S_v1_10163508
- E2611L_v1_10165561
- E2611L_v1_10174216
- E2611S_v1_10173923
- E2611L_v1_10163664
- E2611S_v1_10165560
- E2611S_v1_10171415
- E2611S_v1_10178429
- E2611L_v1_10178428
- E2611S_v1_10182784
- E2611L_v1_10182785
- E2611S_v1_10185504
- E2611L_v1_10185505
- E2611S_v1_10197138
- E2611L_v1_10199957
- E2611S_v1_10201079
- E2611L_v1_10204922
- E2611S_v1_10204921
- E2611L_v1_10211751
- E2611S_v1_10211326
- E2611S_v1_10220818
- E2611L_v1_10221021
- E2611L_v2_10230127
- E2611S_v2_10230126
- E2611S_v2_10236993
- E2611L_v2_10236994
- E2611S_v2_10245967
- E2611L_v2_10252694
Safety DataSheets
The following is a list of Safety Data Sheet (SDS) that apply to this product to help you use it safely.NEBuilder® Positive Control
Gibson Assembly Master Mix
Legal and Disclaimers
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email busdev@neb.com.This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
New England Biolabs (NEB) is committed to practicing ethical science – we believe it is our job as researchers to ask the important questions that when answered will help preserve our quality of life and the world that we live in. However, this research should always be done in safe and ethical manner. Learn more.
Licenses
Limited Warranty: The Gibson Assembly® Master Mix and Gibson Assembly Cloning Kit are warranted to perform according to specifications stated on the certificate of analysis. No other warranty is made, whether express or implied, including any warranty of merchant ability or fitness for a particular purpose. This warranty limits NEB’s and its licensors’ liability to only the price of the product. Neither NEB nor its licensors shall have any responsibility or liability for any special, incidental, indirect or consequential loss or damage whatsoever.
Other Products You May Be Interested In
The supporting documents available for this product can be downloaded below.